| Home | | | News | | | Basics | | | People | | | History | | | Links | | |
Hot Points |
| | FAQ | | |
ATP synthase Basics
Here is a brief description of this wonderful enzyme. If you need more detailed general information see the FAQ list, or try wonderful lecture of A. Crofts (mirrored in University of Hamburg ).
|
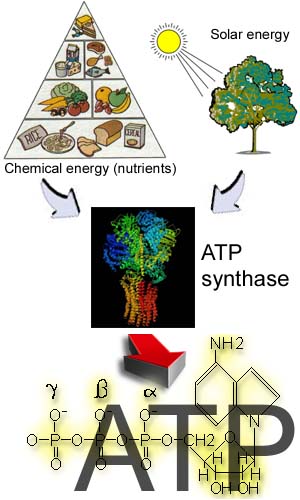
This enzyme is the primary source of ATP
in a vast majority of
living species on Earth, including us. In human body it daily generates
over 100 kg of ATP, which
is subsequently used to provide energy for different biochemical
reactions, including
DNA and protein synthesis, muscle contraction, transport of nutrients
and neural activity, to name just a few. As could be deduced from the name of the enzyme, it catalyses the reaction of ATP synthesis/hydrolysis. The catalytic act is coupled with vectoral transmembrane translocation of several protons. The driving force for ATP synthesis is the transmembrane electrochemical gradient of protons, while during ATP hydrolysis this gradient is built using the energy of ATP phosphodietheric bond.  |
 |
The overall equation is:
ADP3- + HPO42- + H+ + nH+out <=> ATP4- + H2O + nH+in
where indices "out" and "in" denote the outer (positively charged) and the inner (negatively charged) side of the membrane, respectively.
The structure of this enzyme is rather sofisticated. It is an
asymmetric multisubunit protein complex of about 500 kDa. It consists
of two distinct (also in function) multisubunit portions. Hydrophobic Fo
portion is embedded into the membrane and performs proton
translocation, while hydrophillic F1 portion protrudes into
the aqueous phase and performs ATP synthesis/hydrolysis.
During catalysis a complex formed by certain subunits rotate relative
to the rest of the enzyme. This feature makes ATP synthase the smallest
rotary machine ever known.
| Constructed and maintained by Boris A. Feniouk |
Summer 2002 |